Review of Titanium Dioxide: Discussion Paper for Additional Endpoints
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
1. Titanium dioxide (TiO2) was an authorised Food Additive (E171) in the EU and currently remains authorised in the UK, under Retained EU Regulation No. 1333/2008 and Retained EU Regulation No 231/2012. It is used in food as a colour to make food more visually appealing, to give colour to food that would otherwise be colourless, or to restore the original appearance of food. Titanium dioxide has been the subject of multiple safety evaluations.
2. In 2016, EFSA evaluated the safety of E171 and determined that it consisted mainly of micro-sized titanium dioxide particles, with a nano-sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E171 were highlighted, noting that no limits for the particle size of E171 were set. Similarly, with regard to toxicity, uncertainties around the identity and characterisation of E171 were also highlighted.
3. Specifications of E171 titanium dioxide were reviewed again in 2019. Based on the fraction of nanoparticles present in E171, it was determined that the food additive fell under the scope of the EFSA guidance on nanotechnology for “a material that is not engineered as nanomaterial but contains a fraction of particles, less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm”. Thus, a recommendation for re-assessment of the safety of titanium dioxide was proposed and as a result an updated EFSA Opinion was published in May 2021.
4. In this 2021 opinion, the EFSA Panel considered that some findings regarding immunotoxicity and inflammation with E171 as well as neurotoxicity with TiO2 nanoparticles may be indicative of adverse effects. They also considered that there are indications of the induction of aberrant crypt foci with E171 and that no studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available. Overall, on the basis of the currently available evidence along with all the uncertainties, in particular the fact that the concern regarding genotoxicity could not be resolved, the EFSA Panel concluded that E171 can no longer be considered as safe when used as a food additive.
5. Following the publication of the EFSA Opinion, the UK’s COT and Committee on Mutagenicity (COM) considered the EFSA findings, and an interim position paper was published (COT, 2022). Overall, it was observed that the percentage of absorption was reported to be higher in the 2021 opinion than in the previous evaluation (EFSA, 2016), based on the same dataset. Additionally, the COT also questioned the conclusions with regards to the ability of TiO2 to induce aberrant crypt foci. Furthermore, the findings of the studies on neurotoxicity were considered inconsistent by the COT. It was noted that the extended one generation reproduction toxicity study (EOGRT) did not report any effects and that most of the other studies on this endpoint were of nanomaterials. They considered that had the test material in the EOGRT study been dispersed and stabilised in the nano form, some effects could possibly have been observed. The COT, as previously, questioned the relevance of such dispersion to real world use. Members noted that the histopathology tests performed for the EOGRT study were standard and were not sensitive enough in comparison to other studies on this endpoint that performed specific neuro-histopathology testing.
6. With regards to genotoxicity, the COT were in agreement with the COM’s view and further noted the large discrepancy between the underlying dataset and the conclusions drawn by EFSA. They further highlighted the inconsistencies between the outcomes of the 2020 Scientific Committee on Consumer Safety (SCCS) Opinion discussed in detail in paragraph 10, where it was determined that the genotoxic effects of titanium dioxide manifest either via a threshold or secondary mechanism, and the outcomes of the 2021 EFSA evaluation, where the Food Additives and Flavourings (FAF) Panel concluded that it was unclear if a threshold mode of action could be assumed. Regarding the genotoxicity of the nanoparticles, the COT considered that this could either be a concentration effect leading to oxidative damage or a stress effect, however, it was unclear as the results in different cell lines were equivocal and inconsistent. It was also noted that in some test's titanium dioxide had shown less reactivity.
7. On balance, the Committee considered that the weight of evidence did not support the conclusions drawn by EFSA. The COT also agreed with the comments of the COM with regards to risk communication that “As it stands the conclusion is highly risk adverse based on the weak evidence available, and it might create unnecessary concern to the public.” The COT suggested that the COM should independently review the database on genotoxicity and apply their Guidance on determining thresholds. When considering whether they agreed with EFSA’s conclusion that no differentiation could be made with regards to size/form of titanium dioxide and different aspects of toxicity, the COT took the opinion that nanoparticles were driving the toxicity. COM are currently assessing the genotoxicity of TiO2.
8. The full interim position paper is available here: Link to the TiO2 interim position paper. Considering the outputs of the discussions from the COT and the COM, the FSA has decided to launch their own review of the safety of titanium dioxide as a food additive.
9. A previous paper presented to the COT in March 2023 covered the data from an EOGRT study as well as information from the literature. The aim of this previous paper was to present the data underlying the main changes in the 2021 Opinion conclusions on toxicokinetic and absorption data, reproductive toxicity and aberrant crypt foci, developmental immunotoxicity and neurotoxicity from a recent EOGRT study and a revised literature search covering the period from 2015-2021. During this review, Members noted that EFSA had indications that when used by industry, E171 was dispersed into nanoparticles by sonication and therefore COT also considered data on materials made solely of nanoparticles for the assessment. However, this was questioned by COT Members as it was noted that pure nano titanium dioxide would lose its technical function (as it would not provide colour) and would therefore not be of use in food.
10. Also, it was noted that the size and shape of the titanium dioxide particle can affect absorption and agglomeration, however, it was also noted that some studies had some uncertainty about the mode of action. There was evidence that suggested that particles can pass into the blood brain barrier into the placenta via passive diffusion and active uptake. However, it was unclear on what form the titanium dioxide material was when it got into various organs and the duration it stayed there for. It was agreed that there was evidence of absorption but there was little evidence on accumulation reported in studies. The full COT March 2023 Discussion paper is available here: TiO2 March COT 2023 discussion paper. The COT’s conclusion on this discussion paper is available here: COT Minutes March - Final.
11. This current paper presents the data underlying the main changes in the 2021 Opinion conclusions on immunotoxicity and neurotoxicity, COT’s initial conclusions on immunotoxicity and neurotoxicity, and a revised literature search covering the period from 2021-2023 on the following topics: Reproductive Toxicity, Immunotoxicity, Neurotoxicity, Developmental toxicity considerations including ADME (Absorption, Distribution, Metabolism and Excretion), and other toxicological effects.
Background
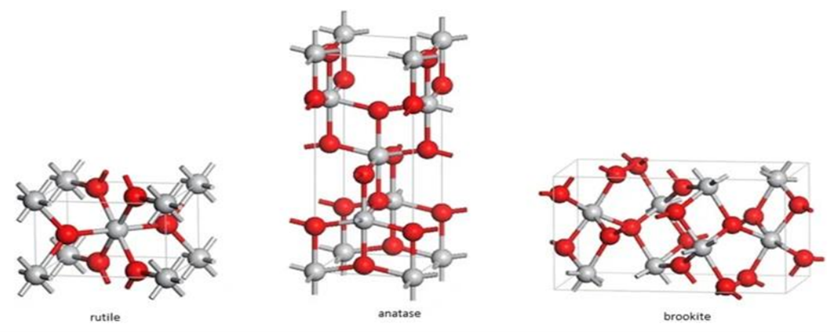
12. Titanium dioxide (TiO2) is an inorganic compound which exists in nature in different crystalline forms – the anatase and rutile being the two most important (see Figure 1).
- Chemical Abstracts Service (CAS) Registry number: 13463-67-7.
- European Inventory of Existing Commercial Chemical Substances (EINECS) number 236-675-5.
- Colour Index (C.I.) number: 77891.
13. Titanium dioxide was an authorised Food Additive (E171) in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 in both anatase and rutile forms (Commision Regulation (EU) No 231/2012) and is still authorised under GB Food Law (retained EU law Regulation No 1333/2008 on food additives).
14. The uses of titanium dioxide include:
- As a colour to make food more visually appealing
- To give colour to food that would otherwise be colourless
- To restore the original appearance of food.
15. It is also widely used in cosmetics and medicines (EFSA, 2016).
2021 Evaluation of TiO2 by EFSA
16. The following section of this paper discusses the EFSA evaluation of TiO2 nanoparticle (NP) studies published between 2016 and 2021.
Neurotoxicological considerations (focus on non-developmental)
Neurotoxicological studies considered by EFSA
17. EFSA concluded that no reliable studies preformed with E171 concerning neurotoxicity were found in the literature (EFSA, 2021). In studies with TiO2 NP >30 nm, after both gestational and lactational exposure, increased hippocampal apoptosis and reduced hippocampal neurogenesis were observed in the offspring (post-natal day (PND) 1 in gestation group, PND 22 in lactation group) of female Wistar rats (n=6/group) exposed from gestational day (GD) 2 to 21 at 100 mg/kg bw/d TiO2 (<100nm) (Ebrahimzadeh et al., 2017; scoring 3 for nanoscale considerations (NSC). At the only dose tested of TiO2 NPs (90 nm- range 40-140nm) 500 mg/kg bw/d for 14 days, male albino rats n=20/group) adverse effects in the central nervous system (CNS) which were possibly related to oxidative stress were reported (Kandeil et al.,2019; NSC score 3). The Panel concluded that “these data show that oral TiO2 NPs administered to rats during embryofetal and early postnatal development reduced hippocampal neurogenesis at 100 mg/kg bw per day, and that oral administration to adult rats produced adverse effects in the brain consistent with oxidative stress at 500 mg/kg bw per day” (EFSA, 2021).
18. In studies using TiO2 NPs < 30 nm, effects were seen at doses as low as 2.5 mg/kg bw per day. This was observed in a study in mice (n = 20/group) dosed by gavage at 0, 2.5, 5 and 10 mg/kg bw/d for 35 days with TiO2 NPs. Reduced volume of the hippocampus and the polymorph layer of the dentate gyrus as well as reduced density and total number of dentate gyrus granular cells were observed even at the lowest dose (Rahnama et al., 2020; NSC score 4). In the Zhang et al., (2020) study (NCS score 3) there were no effects reported on body weight or histopathology of the gut or brain, however a significant decrease in the richness and evenness of gut microbiota, elevated gut HuC/D and TuJ1 and marked reduction of serotonergic markers Sstr1 and Sstr2 in the gut but not in the cerebral cortex. These results suggest an effect on the enteric nervous system, whereas no effects were seen in inflammatory cytokines or gut–brain peptides secreted by endocrine cells and enteric neurons. In an open field test, centre field activity was significantly reduced by the treatment, which is consistent with anxiety-like behaviour, however spatial memory and learning were unaffected in male mice (n = 15/group) dosed at 0 and 150 mg/kg bw/d TiO2 NPs (21 nm) for 30 days.
19. The Panel considered that TiO2 NP (21 nm) at 150 mg/kg bw per day, the only dose tested, altered gut microbiota, without pathological changes in the small intestine and brain.
20. In the Grissa et al., (2016) study, rats were given TiO2 NPs (5 – 10 nm) by oral gavage at the following concentrations: 0, 50, 100, and 200 mg/kg bw per day for 60 days. Reduced brain cholinesterase was found at 100 and 200 mg/kg bw per day (no dose-response), as well as reduced plasma cholinesterase activity at all doses tested (35%, 50% and 50% at 50, 100 and 200 mg/kg bw per day, respectively). Cerebral cortex Glial fibrillary acidic protein (GFAP)-positive cell counts were also dose-dependently increased at 100 and 200 mg/kg bw/d (Grissa, et al., 2016). The Panel noted the methodology of the authors did not indicate whether plasma cholinesterase activity represented acetylcholinesterase or butyrylcholinesterase or both (but considered it was probably both) and noted that TiO2 NPs reduced brain cholinesterase activity.
21. In the Canli et al., (2020) study, rats were given TiO2 NPs (21 nm) at 0, 0.5, 5 or 50 mg/kg bw per day. One death occurred at 0.5 mg/kg bw per day group (no further details reported), but no other notable clinical signs. There was no effect on liver total, reduced or oxidised glutathione (tGSH, rGSH or GSSG) or the ratio between reduced and oxidised glutathione (GSH/GSSG ratio), or on kidney and intestine ATPase activity. Brain Na/K-ATPase activity was significantly increased (approximately 2-fold) at 0.5 and 5 mg/kg bw per day, Mg-ATPase and total ATPase activity at 5 mg/kg bw per day. Brain cholinesterase activity was significantly reduced at all doses (by about 50%, 35% and 50% at 0.5, 5and 50 mg/kg bw per day, respectively, i.e. no dose response). The authors reported that Transmission Electron Microscopy (TEM) demonstrated the presence of TiO2 particles in the liver, kidney and brain which ‘seemed dose dependent.’ The Panel noted that verification of the elemental composition of the particles of interest was not performed in the liver, kidney and brain. This apparent 200-fold difference in potency adds to the uncertainty; possible contributory factors include differences in test substance dispersion and internal exposure between Grissa et al, (2016) and the current study.
22. EFSA noted that, in rats, ‘the most sensitive endpoint was reduced brain cholinesterase activity (about 35-50%) and increased brain Na, K-APTase activity (about 2-fold), observed with TiO2 NPs (21 nm) at all doses tested, in female albino rats dosed for 14 days, as reported by (Canli et al., 2020; scoring 4 for NSC). In this study, rats (n = 6/group) were dosed by gavage with TiO2 NPs (21 nm) at 0, 0.5, 5 or 50 mg/kg bw per day’ (EFSA, 2021).
23. Concerning studies reporting effects on cholinesterase activity the Panel noted that: ‘the most sensitive endpoint in adult rats was reduced (dose related) brain cholinesterase activity and increased brain Na/K-ATPase activity, observed at 0.5 mg/kg bw per day (in females dosed for 14 days), the lowest of three doses tested, reported by Canli et al., (2020) with TiO2 NPs (21 nm). However, Grissa et al., (2016) reported reduced brain cholinesterase activity at 100 but not 50 mg/kg bw per day (in males dosed for 60 days with TiO2 NPs (5–10 nm)). This apparent 200-fold difference in potency adds to uncertainty’ (EFSA, 2021).
Present EFSA Opinions/Conclusions
24. Overall, EFSA concluded that: ‘Overall for neurotoxicity, adverse effects were seen with TiO2 NPs < 30 nm. In adult female rats (Canli et al., 2020; scoring 3 for NSC), adverse effects (reduced brain cholinesterase, and increased brain Na/K-ATPase activity) were observed with TiO2 NPs (21 nm) at 0.5 mg/kg bw per day, the lowest of three doses tested, in a 14-day study’ (EFSA, 2021).
COT initial conclusions
25. In July 2021, the findings of the studies on neurotoxicity were considered inconsistent by the COT. It was noted that the EOGRT study did not report any effects and that most of the other studies on this endpoint were of nanomaterials. Members were advised that in the EFSA evaluation, the issue of the test material in the EOGRT not being dispersed was taken into consideration with regards to the conclusions on this endpoint. They considered that had it been dispersed and stabilised in the nano form, some effects could possibly have been observed. The COT, as previously, questioned the relevance of such dispersion to real world use. Members noted that the histopathology tests performed for the EOGRT study were standard and were not sensitive enough in comparison to other studies on this endpoint that performed specific neuro-histopathology testing (COT, 2021).
Immunotoxicological considerations (focus on non-developmental)
Studies considered by EFSA
26. The findings of studies concerning E171 on immunotoxicity and inflammation were considered inconsistent by EFSA. In mice, one study found no adverse effects at 100 mg/kg bw/d (Riedle et al., 2020; scoring 1 for NSC), while another found a reduction of colonic crypt length, an increase in colon macrophages and CD8 cells in IL-10, TNF-α and IL-6 mRNA at doses of 10 and 50 mg/kg bw/d (Pinget et al., 2019; NSC score 2). At doses of 2 and 5 mg/kg bw/d of E171 in mice increased inflammatory parameters were also observed (Talamini et al., 2019; Urrutia-Ortega et al., 2016). Furthermore, E171 had no effect on the formation of tumours but could enhance intestinal tumour formation in mice exposed to azoxymethane/dextran sulphate sodium (Urrutia-Ortega et al., 2016). In rats, a significant decrease in granulocyte-macrophage colony-stimulating factor (GM-CSF) plasma level (~40%) was observed at the highest dose (10, 100 and 1,000 mg/kg bw/d for 90 days) (Han et al., 2020). It was noted that, ‘GM-CSF is involved in haematopoiesis which may explain the modest but statistically significant decrease in immunoglobulin (Ig) M level (~ 10%)’ (EFSA, 2021). Bettini et al., (2017) (NSC score of 1) found increased inflammatory parameters at the only dose tested (10mg/kg bw/d). This was not observed in a study by Blevins et al., (2019), which reported no effects up to 267 mg/kg bw/d. The Panel noted that this study scored 3 for NSC. Pages 115-120 of the EFSA opinion provide further details on the methodology and findings.
27. In gavage studies with TiO2 NPs > 30 nm (Table 1) effects were seen at a dose of 20 mg/kg bw per day in rats (90 days, dosed at 20 and 40 mg/kg bw/d) in a study by Hashem et al., 2020. Two mice studies, following dosing with TiO2 NPs for either 5 or 7 days at 5, 50 and 500 mg/kg bw/d, reported inflammatory responses seen in the stomach even at the lowest dose tested (Mohamed, 2015; Li et al.,2019). No significant histopathological changes were observed in a study with 1 mg/kg bw/d TiO2 NPs (25, 50 or 80 nm) investigating effects on the histology of the spleen (Chen et al., 2015). In studies with TiO2 NPs < 30 nm effects were observed at doses as low as 2.5 mg/kg bw per day in mice.
Table 1: Immunotoxicity studies with TiO2 NPs <30 nm.
|
Test System |
Exposure |
Characterisation of test substance |
Results |
EFSA’s evaluation |
Ref |
|
Female CD-1 (Institute for Cancer Research (ICR) mice (n=20/group) |
Gavage: 2.5, 5 and 10 mg/kg bw/d for 90 days |
TiO2 NPs (5-6 nm) |
Inflammatory lesions and damage of cardiac tissue histopathologically- more pronounced at mid and high doses. Expression of NF-κB, and of pro-inflammatory cytokines TNF-α ,IL-1β, IL-6 and IFN-γ were increased in a dose-dependent fashion (statistically significant increase up to 1.8-fold compared with the control); expression of the NF-κB inhibitor I-κB was decreased in a dose-dependent fashion (statistically significant decrease up to1.55-fold compared with the control), as evidenced by western blotting. |
The Panel considered that these data indicate an effect of TiO2 NPs (5– 6 nm) exposure at all dose levels tested, as evidenced by histopathological lesions, corroborated by intermediate endpoints indicating disturbance of intracellular ion homeostasis that were adrenergic receptors in the heart. These lesions are accompanied by increases in the expression of intermediate inflammatory endpoints. The Panel noted effects on inflammatory mediators with TiO2 NPs (5–6 nm) at all doses tested and corroborated by histopathological lesions. |
Yu et al., 2016 |
|
Male C57BL/6 mice (n=10/group) |
Gavage: 100 mg/kg bw/d for 28 days |
TiO2 NPs (20 nm) anatase;
TiO2 NPs (15 nm) rutile |
No effects on body weight.
Particles observed in spleen however, no histopathological changes. No histopathological changes in lung, jejunum, kidney, liver, brain.
Increased length of villi in colon, irregularly arranged epithelial cells.
Rutile NPs had a more pronounced influence on the gut microbiota than anatase NPs. The most influenced phylum was Proteobacteria, which was significantly increased by rutile NPs but not by anatase NPs. At the genus level, Rhodococcus was enriched by rutile NPs, Prevotella was significantly decreased by both forms of the TiO2 NPs. |
The Panel considered that these data support an effect of TiO2 NPs on the microbiota, but as no immunological parameters other than the histopathology of the spleen were included in this study, any consequence(s) associated with these changes in terms of inflammation and the immune system remain uncertain. |
Li et al., 2018 |
|
C57BL/6J mice (n=30/group) |
Gavage: 150 mg/kg bw/d for 30 days |
TiO2 NPs (21nm) |
Significantly changed richness and composition of the gut microbiota. No changes in parameters indicating inflammation (IL-6 and IL-1b) in either intestines or brain were observed. |
The Panel considered that exposure to TiO2 NPs (21 nm) leads to changes in the microbiota composition, but the study does not indicate a local or systemic inflammatory action. |
Zhang et al., 2020 |
|
Sprague Dawley rats (n=10/group) |
Gavage: 0, 2, 10, 50 mg/kg bw/d, glucose (1.8 g/kg bw/d) TiO2 NPs (0, 2, 10 and 50 mg/mg bw /d) + glucose (1.8 g/kg bw per day) for 30 or 90 days. |
TiO2 24nm |
No significant histopathological changes were observed in the spleen in all groups.
Increases in white blood cell counts and granulocyte in female rats after exposure to TiO2 NPs 50 mg/kg bw/d for 90 days and in male rats exposed to TiO2 NPs 50 mg/kg bw per day for 30 days: increase in white blood cell counts, lymphocytes, monocytes absolute numbers and in the percentage of lymphocytes and granulocytes. At 90 days an increase in percentage of monocytes was measured.
Decrease in the white blood cells at 90 days in rats exposed to 10 mg/kg bw/d. |
The Panel considered that the increase in leucocytes may suggest an inflammatory response induced by TiO2 NPs (24 nm) at the highest dose tested (50 mg/kg bw per day). |
Chen et al., 2015a |
|
Sprague Dawley rats (n=6/group) |
Gavage: 0, 2, 10, 50 mg/kg bw/d for 30 days |
TiO2 NPs (29 nm) |
Histopathologically, reduced numbers of goblet cells were found as a result of exposure, as well as inflammatory infiltration, while in serum increased IL-6 expression was observed. |
N/A |
Chen et al., 2019 |
|
Male Wistar rats (n=8/group) |
Gavage: 0, 50, 100, 200 mg/kg bw/d (5 times/week for 8 weeks) |
TiO2 NPs (5- 12nm) |
Statistically significant dose-related increase in the level of NO in 100 and 200 mg/kg bw/d TiO2 NPs groups was observed together with a statistically significant increase in brain TNF-α in 200 mg/kg bw/d TiO2 NPs group. The increase was dose-related for both parameters. |
The Panel noted changes for the above-mentioned inflammatory markers at doses starting from100 mg TiO2 NPs (5–12 nm)/kg bw per day. |
Grissa et al., 2020 |
Present EFSA Opinions/Conclusions
28. EFSA concluded ‘that these studies indicate immune deregulatory activity by E 171, evidenced by several immune-related and inflammatory markers. These effects were not observed up to 50 mg E 171/kg bw per day. In three single dose level studies with E 171, effects were noted at lower doses, i.e. 2, 5 and 10 mg/kg bw per day. Effects of E 171 may, at least in part, stem from the activity of the fraction of the smaller TiO2 particles, as studies with these particles also indicate inflammatory effects of exposure to TiO2 NPs (5–6 nm) at 2.5 mg/k g per day’ (EFSA 2021).
COT initial conclusions
29. The COT noted that in several parts of the Opinion, published papers were presented at face value, and there was no discussion of the results nor the overall Weight of Evidence to support the conclusions being made. They furthermore noted discrepancies and conflicts between the results of the studies reported and the overall conclusions. Overall, the COT considered that there appeared to be a lack of internal consistency and of objective weighing of all the evidence (COT, 2021).
Literature review 2021-2023 – Studies since the EFSA (2021) evaluation
30. An updated literature search was carried out for papers published from 2021 to 2023 on the following topics: Reproductive Toxicity, Immunotoxicity, Neurotoxicity, Developmental toxicity considerations including ADME (Absorption, Distribution, Metabolism and Excretion), and other toxicological effects. Parameters for the literature search can be found in Annex A. Thirty-two studies from the literature search were considered relevant.
Reproductive Toxicity
Ni, et al., 2021 – Non-sonicated study
31. The mechanism by which TiO2 NPs cross the blood-testis barrier (BTB) was investigated by treating TM-4 cells with various concentrations (0, 6.25, 12.5, 25, 50, 75 and 100 μg/mL) of TiO2 NPs (3 nm and 24 nm) for 24 h.
32. TM-4 cells were identified as an appropriate in vitro Sertoli cell model of BTB. Cell viability, apoptosis, oxidative damage, as well as the expression levels of actin regulatory and tight junction (TJ) proteins were assessed in TM-4 cells treated with TiO2 NPs (3-nm and 24-nm).
33. The cytotoxicity of 3 nm TiO2 NPs was greater than that of 24 nm TiO2 NPs. Both 3 nm and 24 nm TiO2 NP treatments induced significant oxidative stress, decreased the expression of TJ proteins (occludin, ZO-1 and claudin 5), damaged the TJ structure, and exhibited enlarged gaps between TM-4 cells. 3 nm TiO2 NP-treated cells showed increased cytotoxicity and decreased Annexin II expression, while 24nm TiO2 NP-treated cells showed increased Arp 3 and c-Src expression.
34. Decreased catalase activity was observed in TM-4 cells treated with 30 μg/ml TiO2 NPs for 24 h, whereas no significant change in CAT activity was observed upon exposure to 60 μg/ml of either TiO2 NP size. In TM-4 cells treated with both sizes of TiO2 NPs, hydrogen peroxide levels were enhanced, and no apoptosis was observed.
35. Taken together, the results of this study showed that the toxicity of TiO2 NPs to TM-4 cells was both concentration- and size-dependent. The higher TiO2 NPs concentration showed greater toxicity to TM-4 cells. Meanwhile, the smaller sized TiO2 NPs presented higher toxicity to TM-4 cells. In conclusion, these results indicated that TiO2 NPs of both sizes crossed the BTB by disrupting actin-based adhesive junctions of TM-4 cells, affecting spermatogenesis.
Yao, et al., 2021 – Unknown sonication
36. To investigate the effects of TiO2 NPs in the blood-milk barrier of maternal rats and the growth of offspring, pregnant female Sprague Dawley rats (7 per group) were administered with TiO2 NPs (dose of 100 mg/kg of bw/day) by oral gavage from postnatal day (PND)1 to PND20.
37. The findings illustrated the deleterious pathological effects of oral exposure to TiO2 NPs in dams and developmental concerns in the offspring. The results indicated that exposure to TiO2 NPs could cause severe pathological damage to the mammary glands. The accumulation of TiO2 NPs in rat mammary glands caused no nutritional changes to the breast milk but the NPs could be transferred to offspring through an impaired blood-milk barrier. Also, the accumulation of TiO2 NPs in newborns was seen to negatively influence their growth and development. Notably, it was found that the oxidative stress was the predominant toxic mechanism associated with impaired blood-milk barrier system following exposure of TiO2 NPs.
Deng, et al., 2022 – Sonicated study
38. TM4 Sertoli cells (cells isolated from a mouse testis) were cultured and treated with different concentrations of TiO2 NPs (0, 50, 100, 150, 200 μg/mL).
39. The TiO2 NPs used in this study was 99.8% anatase, with particle sizes between 14-21 nm. Before application, the TiO2 NPs concentrations were vibrated by ultrasonic oscillation for 30 min to prevent particles from aggregation and then diluted to the desired concentrations.
40. A significant decrease in the cell viability in TM4 cells was seen after exposure to TiO2 NP exposure at the concentrations of 100, 150, and 200 μg/mL. It was also found that exposure to TiO2 NPs activated the JNL signalling pathway in the TM4 cells, inducing mitochondria-mediated apoptosis of cells. The excessive apoptosis led to abnormal expression of BTB junction proteins (ZO-1, Claudin-11, and β-catenin), which can result in male sterility.
Said, et al., 2022 – Sonicated study
41. Adult male rats were injected with TiO2 NPs (100 ppm a day) for 60 days to assess its reprotoxic impacts.
42. The TiO2 NPs were sonicated during the preparation phase and then found to be spherical with a small aggregation, with an average particle size of 85 nm.
43. After 60 days, hormonal assays, semen appraisal, lipid peroxidation, antioxidant enzymes, testis and prostate morphometry, and the steroidogenesis-related genes’ mRNA expressions were assessed. Results showed a significant decrease in sperm motility and concentration, reduced sex hormone levels, altered the testicular oxidant/antioxidant status and mRNA expression of steroid-related genes, and induced architectural alterations in testicular, epididymal, and prostate gland tissues.
Kamal, et al., 2023 – Non-sonicated study
44. The effects of prenatal exposure to chemical TiO2 NP (CHTiO2 NPs) and green-synthesised TiO2 NPs (GTiO2 NP) on immunological and oxidative status as well as lungs and spleen was investigated in pregnant female rats and their foetuses. The TiO2 NPs used showed agglomeration with a spherical or irregular spherical shape.
45. The pregnant mice (10 mice/group) were divided into groups receiving the following doses: distilled water (control/ group 1), 100 mg/kg bw CHTiO2 NPs (group 2), 300 mg/kg bw CHTiO2 NPs (group 3), 100 mg/kg bw GTiO2 NPs (group 4), and 300 mg/kg bw GTiO2 NPs (group 5). The pregnant mice received these doses by daily oral gavage for 14 days beginning around the time of implantation (on a gestational day (GD) 6 until GD 19).
46. The serum level of proinflammatory cytokines IL-6, oxidative stress markers (MDA and NO), and antioxidant biomarkers (superoxide dismutase (SOD) and GSH-PX) were assayed.
47. Significant increases in proinflammatory cytokines IL-6 levels were seen in mice given 300 mg/kg bw CHTiO2 NPs and both GTiO2 NP concentrations.
48. NO levels and MDA activity were found, as well as significant decreases in GSH-Px and SOD activities, revealing its oxidative effect. In both CHTiO2 NP treated groups, there were significant increases in MDA activity and significant decreases in GSH-Px and SOD activities, suggesting an oxidative effect. Meanwhile, significantly increased levels of GSH-Px and SOD activities were seen in mice given 300 mg/kg bw GTiO2 NPs, strongly indicating the antioxidant effect of GTiO2 NPs.
49. Furthermore, histopathological findings of the spleen and lungs of the CHTiO2 NP treated groups revealed severe congestion and thickening of the blood vessels, whereas GTiO2 NP treated mice only had mild tissue alterations.
Arslan, et al., 2022 - Unknown if sonicated
50. The Arslan et al. study investigated the effect of Ti02 NPs and silver nanoparticles (Ag NPs) had on the mitochondrial dynamics in mouse testis tissue. The study involved three groups of mice, with ten mice in each group: a control group, a TiO2 NP group (5 mg/kg per dose), and an Ag NP group (5 mg/kg per dose).
51. The NPs were administered in 5 doses over 15 days (with three-day intervals between doses) to the mice via the tail vein. The study analysed the expression levels of genes related to mitochondrial fusion (Mfn1, Mfn2, OPA1) and fission (Drp1) to determine the potential toxic effects of the NPs on mitochondrial dynamics. The results showed that both Ag NPs and TiO2 NPs were able to enter the testis tissue via the blood-testis barrier and accumulate there.
52. The study found that the administration of Ag NPs did not have a significant toxic effect on the testicular weight, testicular index, or sperm parameters. Additionally, the study did not detect any disruption of mitochondrial dynamics in testicular cells in response to Ag NP treatment.
53. However, the administration of TiO2 NPs (anatase, <25 nm) led to a decrease in sperm motility and the percentage of sperms with swollen tails. Moreover, the study found that TiO2 NPs disrupted mitochondrial dynamics in testicular cells by causing an excess of mitochondrial fission, as evidenced by the excessive expression of the Drp1 gene and DRP1 protein.
54. This study is the first to report on the potential toxicity of nanoparticles on mitochondrial dynamics in testicular cells, specifically the mechanisms of mitochondrial fusion and fission.
Al-Doaiss, et al., 2021 – sonicated study
55. To investigate if TiO2 NP exposure might induce acute damage in the heart and testis, male Wistar albino rats (10 rats/group) were intraperitoneally given a single dose of TiO2 NPs (126, 252, 378 mg/kg bw).
56. The TiO2 NPs (99.7% with an average particle size of 25 nm) were dispersed in normal saline and sonicated for 15 min.
57. In a dose dependent manner, exposure to TiO2 NPs resulted in cardiac alterations, including myofibers wavy appearance, myofiber disarray, partial cross striation, cardiomyocytes hydropic degeneration together with vacuolation and nuclear alterations. Acute exposure to TiO2 NPs also resulted in spermatocyte degeneration, spermatid sloughing and testicular oedema.
Danafar, et al., 2021 - Sonicated study
58. The impairment of sperm efficacy in mice following short-term nano-titanium dioxide exposure was assessed. In this study, thirty-five NMRI male rats were exposed to TiO2 NPs (anatase/rutile, 99+%, 20 nm) which was previously dissolved in water and homogenised with a sonicator for 10 minutes. The TiO2 NP suspension was then prepared at concentrations of 2.5, 5 and 10 mg/kg. The mice were then divided into four groups (n = 8/each group), including a control group and three treated groups. The control group received water intragastrically. The group I, group II and group II treated groups were fed orally with 2.5, 5 and 10 mg/kg/day TiO2 NPs for 40 days, respectively.
59. After 40 days, sperm parameters, DNA integrity and chromatin quality were assessed using chromomycin A3, aniline blue, toluidine blue staining and terminal deoxynucleotidyltransferase dUtp nick end labelling (TUNEL). Hematoxylineosin staining was performed to measure spermatogenic cells and the total diameter of seminiferous tubules. Also, sex hormone and malondyaldehyde levels were measured.
60. It was found that Abnormal sperm tails rose in group III (28.87 ± 4.91) in comparison with the control group (12.75 ± 3.95). However, chromomycin A3 staining and TUNEL showed higher levels in group III in comparison with the control group, whereas aniline blue and toluidine blue staining showed no differences. A significantly lower spermatogenesis index and lumen parameters were observed in group III. Leydig cell numbers, cellular diameters and the area of the seminiferous tubules were lower in the treated groups. The testosterone level was also lower in these groups and the percentage of malondyaldehyde in the seminal fluid was higher.
61. It was found that the overexpression of the Mitogen-activated protein kinase (MAPK) signal pathway after TiO2 NP exposure disrupted the structure and function of the BTB, which may damage spermatogenesis. It was also stated that TiO2 NPs exposure likely increased oxidative stress, affecting testicular function by activating the MAPK signalling pathway and disrupting the BTB, leading to male reproductive dysfunction. However, it was highlighted that the exact TiO2 NP mechanisms were not clear; and it was emphasised that they may relate to oxidative stress. In conclusion, the authors advised that given their widespread use, TiO2 NPs should be a public health focus of attention.
Mancuso, et al., 2022 – Sonicated study
62. Any adverse effects of TiO2 NPs on the function and viability of porcine prepubertal Sertoli cells (SCs) were investigated in this in vitro study.
63. Porcine prepubertal SCs underwent acute (24 h) and chronic (1 - 3 week) exposures at both subtoxic (5 µg/ml) and toxic (100 µg/ml) doses of TiO2 NPs. The control group was the unexposed SCs (0 TiO2 NPs µg/ml).
64. Anatase TiO2 NPs were used, and TiO2 NP stock solutions were sonicated for 30 min to limit the formation of nanoaggregates.
65. At each time point, SCs exposed to 100 µg/ml TiO2 NPs showed morphological and ultrastructural modifications, whereas no substantial morphological changes were seen in SCs exposed to 5 µg/ml TiO2 NPs at any time point, with ultrastructural alterations appearing only at the third week. Both doses induced the activation of caspase-3 at the first and second week whereas markers of SC functionality (anti-Müllerian hormone (AMH) and inhibin B gene expression) were significantly decreased up to the third week. A marked increase of intracellular ROS and DNA damage was seen at all exposure times in SCs when exposed to the high dose of TiO2 NPs.
66. Increased gene expression of antioxidant enzymes (SOD and heme oxygenase) was observed at both doses, whereas at the highest dose clear proinflammatory stress was measured along with the steady increase in the gene expression of interleukin-1a and interleukin 6.
67. An increased phosphorylation ratio of p-ERK1/2 was observed up to the second week at both doses, followed by the increased phosphorylation ratio of p-NF-kB in the chronic exposure.
68. This study concluded that exposure to 100 µg/ml TiO2 NPs highlighted its negative effects on the morphological-ultrastructural integrity, apoptosis, alteration of functionality, and the induction of a proinflammatory state in SCs. Further, while SCs receiving the 5 µg/ml dose appeared to maintain morphological integrity, an in-depth analysis showed that the SCs were induced to activate a series of responses to neutralize the effects prompted by long time constant TiO2 NP exposure.
Immunotoxicity
Murugadoss, et al., 2021 – Unknown if sonicated
69. The biological effects (cell viability, cell proliferation, oxidative stress, pro-inflammatory response, and DNA damage) of two sonicated TiO2 NP (17 nm and 117 nm) agglomerates of various sizes (small and large agglomerates) in human bronchial epithelial (HBE), colon epithelial (Caco2), and human monocytic (THP-1) cell lines repeatedly exposed to a non-cytotoxic dose (0.76 µg/cm2) were assessed in a three-week study. It was found that neither of the two TiO2 NPs nor their agglomeration states induced any effects (compared to control) in any of the cell lines tested.
Neurotoxicity
Gerber, et al., 2022 – Sonicated study
70. Cultures of rat primary cortical cells were used to investigate in vitro effects of TiO2 NP exposure on neuronal function following acute (0.5 h), sub-chronic (24 h and 48 h), and chronic (14 day) dosing. Acute and sub-chronic exposures used a dose of 30 ug/mL while chronic exposure used a dose of 100 ug/mL. TiO2 average particle size was 26.2 ± 10.7 nm, with a mean diameter 761.8 ± 7.95.2 SD and mode diameter 223.4 ±1.7 SD. The TiO2 NPs were found to have bulky agglomerates in suspension exceeding 1000 nm. During preparation, TiO2 NP stock suspensions were sonicated for 15 min.
71. Acute and sub-chronic exposure to TiO2 NPs did not affect neuronal activity, depolarization-evoked Ca2+ influx or viability, whereas chronic exposure only modestly reduces neuronal function without affecting morphology. The combined results indicate that TiO2 NP exposure is of limited hazard for neuronal function.
Sofranko, et al., 2021 – Non-sonicated study
72. Neurotoxic effects of TiO2-P25 (anatase to rutile ratio of 85:15) in C57BL/6J mice were investigated in a 28-day oral exposure study. Behavioural effects were evaluated by X-maze, open field, string suspension and rotarod tests. Histological alterations were analysed by immunohistochemistry, and brain tissue homogenates were investigated for markers of oxidative stress, inflammation and blood-brain barrier disruption.
73. TiO2-P25 had a mean particle size of 26.2 ± 10.7 nm, mode diameter dmode = 21.0 nm and a nearly spherical particle morphology. It should be noted that P25 is not considered as food additive and thus not equal to food-grade TiO2.
74. Ten male and 10 female mice were fed a diet of food pellets dosed with 10 mg/g TiO2 for 28 days, which resulted in an estimated daily consumption of TiO2 of 2 g/kg bodyweight. Male and female mice showed no significant differences of markers of inflammation, oxidative stress or blood-brain barrier integrity following TiO2-P25 exposure. There were also no consistent significant treatment-related effects on anxiety and cognition. This study concluded that subacute exposure to foodborne TiO2 does not cause marked neurotoxicity in mice.
Cui, et al., 2021 – Sonicated study
75. Effects of TiO2 NPs during the adolescent period on neurotoxicity in Sprague Dawley rats were investigated. Over 31 days, the rats were injected once every two days with 20 mg/kg TiO2 NPs.
76. TiO2 NPs were suspended in 0.9% sodium chloride at a concentration of 10 mg/ml and treated with ultrasound for more than 1 h before use.
77. Memory ability and anxiety-like behaviour were assessed, and endogenous antioxidant state and inflammatory parameters monitored. Significantly increased catalase activity, glutathione peroxidase activity, and total antioxidant capacity were found in TiO2 NP-treated rats, as well as significantly decreased malondialdehyde levels. After exposure to TiO2 NP, anxiety-like behaviour and cognitive impairment were seen in these adolescent rats, likely associated with the neuroinflammation and oxidative stress in the hippocampus also found.
Naima, et al., 2021 – Sonicated study
78. Male Wistar rats (12 rats/group) were injected intravenously with a single dose of TiO2 NPs (20 mg/kg body weight) and were subjected to cognitive and emotional tests to investigate the effects of TiO2 NPs on the behavioural performance, monoamine neurotransmitters and oxidative stress in the rat brain.
79. TiO2 NPs were 20-30 nm and during preparation the TiO2 NP solutions were ultrasonicated for 60 minutes.
80. The emotional reactivity and cognitive capacity of TiO2 NP dosed rats were significantly affected compared to control groups. Along with significantly increased dopamine and decreased serotonin levels, TiO2 NPs also induced oxidative stress in the brain, manifested by increased levels of H2O2 and malondialdehyde, which is associated with disturbances in antioxidant enzyme activities, in particular, SOD and catalase activities.
81. The brain tissue of control rats showed normal architecture, whereas TiO2-treated rats were found to have histological damages in the brain tissue with abundant lymphocytic clusters, capillary dilations, vascular congestion and oedema.
Developmental Toxicity Considerations Including ADME (Absorption, Distribution, Metabolism and Excretion)
Sitia, et al., 2022 – Non-sonicated study
82. Eight-week old male CD1 mice (9 mice/group) were injected with 6 mg/kg body weight of food-grade TiO2 (99.3% anatase) suspended in 200 µL of injection-grade distilled water. Then, at 1, 24, and 168 h after E171 administration, mice were euthanized (3 animals per time point).
83. It was investigated, using TEM analysis, whether serum affected the morphological characteristics of TiO2 suspended in either water or murine serum and incubated for 1, 4, and 24 h at 37 °C. Throughout, TiO2 NPs suspended in water was homogenously distributed with single-particle differentiation however TiO2 NPs suspended in serum formed large compact clusters (0.5–1 µm) regardless of the incubation time, making it almost impossible to discriminate single NPs.
84. Ti blood concentrations peaked one hour after injection, representing about 1% of the injected dose, and then decreased by ~60% after 4 hours and remained low for up to 168 hours. TiO2 NP solutions were not sonicated so to created realistic conditions of use for food-grade TiO2 NPs.
85. Low TiO2 concentrations were detected in liver, spleen, and kidney samples, as well as a decreasing concentration of TiO2 detected in brain samples between 1 and 24 h after injection. An approximately 6-fold increase of detected Ti accumulation in the lungs was also observed at 168 hours.
86. Total numbers of circulating WBC, platelets, lymphocytes, and neutrophils were not affected by E171 administration, whereas the number of monocytes increased significantly.
87. No significant differences in the levels of alanine aminotransferase (sALT), aspartate aminotransferase (sAST), and lactate dehydrogenase (LDH), used as toxicity biomarkers, were observed in animals treated with E171 compared to vehicle-treated mice, indicating that Ti accumulation in organs did not induce overt toxicity.
88. TiO2-treated mice exhibited small microgranulomas after one hour, which then became less pronounced after 24 h, and had completely disappeared 6 days later, suggesting that TiO2 NPs can accumulate in liver parenchyma in small amounts.
89. After TiO2 injection, Ti levels in the liver peaked within the first 24 h and returned to basal levels after 7 days, suggesting that the TiO2 NPs accumulated in the liver can be efficiently cleared out, causing a low and transient toxicity with no signs of chronic hepatic disease.
90. After immunostaining lung samples with the F4/80 antibody to detect macrophage accumulation, interstitial inflammation with F4/80+ cell recruitment was weak at the earliest time points and increased with Ti accumulation over time. One hour after TiO2 injection, there was no evidence of lung parenchyma F4/80 staining, while 24 h after morphological alterations in lung tissues were observed. These alterations were further enhanced in sections from animals euthanized 168 h after injection. At the same time point, lungs of TiO2-treated mice displayed significantly more interstitial immunoreactivity in the alveoli compared with those injected with the control vehicle.
Korábková, et al.,2021 - Sonicated study
91. The behaviour of titanium dioxide (TiO2) particles of rutile, anatase, and their commercial mixture were exposed to various environments, including simulated gastric fluids and human blood plasma (both representing in vivo conditions), and media used in in vitro experiments. The cytotoxicity and the behaviour of TiO2 particles in water and in cultivation media were also examined.
92. In this study, titanium (IV) oxide particles with various sizes and crystallographic phases were used in this study as seen below in Table 2.
Table 2. Specificiation of TiO2 particles used in the study.
|
Sample |
Specific Surface Area (m2/g] |
Crystalline Form |
Crystal size (nm) |
|
Rutile 1 |
76.02 |
Rutile |
21 |
|
Rutile 5 |
7.11 |
Rutile |
150 |
|
Anatase |
45-55 |
Anatase |
<25 |
|
Anatase/Rutile |
n.p. |
Anatase/rutile |
<100*, <50** |
*based on specific surface area of samples as determined by Brunauer-Emmett-Teller (BET) analysis.
**based on X-ray diffraction.
93. TiO2 dispersions were prepared by homogenising 0.05 g TiO2 particles in 10 mL water for 30 minutes using a sonicator. The dispersions where then mixed with simulated saliva, simulated gastric fluids, simulated intestinal fluid and human blood plasma.
94. The study presented a unique comparison of the four different types of TiO2 particles with respect to their behaviour in media used for biocompatibility testing, simulated fluids of the gastrointestinal tract, and plasma.
95. The results showed that all tested forms of TiO2 particles agglomerated immediately after contact with PBS (Phosphate-buffered saline) and serum-free DMEM due to the presence of electrolytes that screen particle surface charge, with agglomeration levels increasing in the following order: Rutile 5 < Anatase < Anatase/Rutile < Rutile 1. However, the presence of calf serum in Dulbecco’s Modified Eagle Medium (DMEM) significantly reduced agglomeration as a result of the formation of a protective protein corona around the particle surface. The authors stated that the general behaviour of TiO2 in media routinely used for biological testing, and the positive influence of serum in DMEM in reducing agglomeration in the medium was demonstrated in this study.
96. To mimic the digestive process, TiO2 particles were exposed to simulated saliva, stomach, and intestinal fluid, suitably supplemented with the digestive enzymes pepsin or pancreatin. The results demonstrated that, contrary to the protective action of calf serum in DMEM, pepsin and pancreatin (though being of a protein nature) triggered the significant agglomeration of TiO2 particles in SGF (Simulated Gastric Fluid) and SIF (Simulated Intestinal Fluid). The formation of an agglomerate was notably higher in comparison to the absence of enzymes. After mixing TiO2 particles with saliva, the samples behaved similarly as in PBS and DMEM with respect to the order of agglomeration level. When mixed with blood plasma, however, TiO2 agglomeration was significantly reduced due to the protective protein corona hindering interactions between TiO2 particles.
97. It was concluded that the crystalline forms of TiO2 particles, ionic strength of surrounding media, and presence of proteins are all factors that influence colloidal stability of TiO2 particles in the media discussed above. Cytotoxicity data determined with NIH/3T3 mouse embryonic fibroblast cells demonstrated an effect dependent on the type of tested TiO2 particles. The lowest cytotoxic effects were recorded for Rutile 1 and the Anatase/Rutile mixture, which were cytotoxic at TiO2 particle concentrations of 4000 and 3500 µg/mL, respectively. Higher cytotoxicity was yielded by Anatase and Rutile 5, which were cytotoxic at 1500 µg/mL.
Violetta et al., 2023
98. The interaction of different TiO2 NP shapes and host tissues after systemic injection in healthy immunocompetent and specific pathogen-free (SPF) mice. In this study, parameters such as biodistribution, accumulation, and toxicity were assessed in the lungs and liver of mice.
99. The three types of TiO2 NP shapes were TiO2 bipyramids (size distribution: 7.5 ± 0.7 nm side; 27.8 ± 2.1 nm length, TiO2 rods (size distribution: 6.3 ± 1.0 nm width; 13.6 ± 2.1 nm side, and TiO2 plates (size distribution: 4.5 ± 0.3 nm thickness).
100. Eight-week old male CD1 mice were randomly divided into three groups receiving TiO2 bipyramids, plates, and rods, respectively (n = 9 for each experimental group). Briefly, all animals received the same dose of each NP preparation (6 mg/kg), diluted in 200 µL of injection grade distilled water, by intravenous injection. At the selected timepoints (pre injection, 1, 12, 24, 96, and 168 h), mice were anesthetized and blood was taken by retro orbital bleeding for complete blood counts, and the serum analyzed for markers of toxicity. Furthermore, at 1, 24, and 168 h after NP injection, three mice for each group were killed and their organs collected for histological analysis.
101. The data showed that the organ accumulation of TiO2 NPs, measured by ICP-MS, were quite low, and this was described as being only partially and transiently affected by the NP geometries. It was stated that the long lasting permanence was exclusively restricted to the lungs. It was also found that rods lead to a stronger and more severe biological effect in comparison to bipyramidal and spherical geometries. Overall, it was concluded that small physico-chemical differences can dramatically modify both accumulation and safety.
Han, et al., 2020 – (Non-peer reviewed study)
102. The toxicity of food-grade TiO2 NP also known as E171 was explored in vivo and in vitro by assessing the sub chronic toxic responses of TiO2 NPs in a 90 day study. The TiO2 NPs were reported to be anatase, with a mean particle diameter was about 150 nm.
103. Five-week old male and female specific-pathogen-free Sprague Dawley rats were given daily doses of either 0, 10, 100, or 1000 mg/kg by oral gavage. No significant tissue damage found at any dose level but when administered with 1000 mg/kg, TiO2 NP were found to penetrate and accumulate in the rat's stomach walls and blood IgM and GM-CSF levels were significantly lower than control rats. Altered antioxidant SOD expression levels were also found.
104. A cancerous human stomach epithelial cell line (AGS) was also used to investigate potential toxic mode of action of TiO2 NP. The findings showed that TiO2 NP also penetrated the plasma membranes of AGS cells.
105. E171 concentrations were measured in the colons, kidneys, and spleens harvested from all rats at necropsy. The findings showed that the concentration clearly increased only in the colons of both sexes administered 1,000 mg/kg E171 compared with the control, indicating that the colon is the main excretion route. In addition, it was also found that E171 accumulated in the cytosol and nuclei of various cells comprising the colon tissue and forms lamella-like structures in the colon tissues.
106. Additionally, it was found that titanium accumulation can also affect the distribution of elements cross-binding with it or participating in the antioxidant response, and it was found that the colonic zinc concentration increases in female rats exposed to E171 compared with the control. It was expressed that, SOD played a central role in inhibiting xenobiotic-induced oxidative damage and subsequent apoptosis. Therefore, the effects that E171 had on SOD-1, SOD-2, and cytochrome C protein in the colonic tissues of rats were assessed. It was found that the expression of SOD-1 and SOD-2 protein were down-regulated in the colonic tissues of both sexes and female rats, respectively. However, cytochrome C expression did not significantly differ among treatment groups.
107. The effects that E171 had on the systemic immune system was also assessed. The Granulocyte-macrophage colony-stimulation factor (GM-CSF) and immunoglobulin (IgM) concentrations were measured in blood samples. It was found that the levels of GM-CSF (female) and IgM (both sexes) significantly reduced in rats administered with 1,000 mg/kg E171 for 90 days compared with the control, whereas there were no differences among treatment groups in terms of their IgG, IgA, or IgE levels. The GM-CSF levels were 46.3 ± 12.1 pg/mL and 27.3 ± 9.3 pg/mL for the female rats in the control and maximum-dose groups, respectively. The IgM levels were 2,123.6 ± 176.3 ng/mL and 1,926.6 ± 77.3 ng/mL in the male and female rats of the control group, respectively, whereas they were 1,886.9 ± 87.7 ng/mL and 1,696.5 ± 152.7 ng/mL for the male and female rats in the maximum-dose group, respectively.
108. Overall, these findings suggest that chronic TiO2 NP intake might negatively affect the host’s defence function against foreign bodies by decreasing antioxidant capacity.
Chen, Han, et al., 2022 – Sonicated study
109. Lipidomic metabolites were investigated in gut-liver axis of Sprague-Dawley rats (7 rats/group) after oral gavage exposure to TiO2 NPs (0, 2, 10, 50 mg/kg−1 bw/day) for 90 days.
110. TiO2 NPs mixtures were sonicated for 15 mins and vortexed for 5 mins before each treatment of animals. TiO2 NPs (29 ± 9 nm) were spherical and anatase crystals.
111. Changes in lipidomic signatures of main organs or systems in the gut-liver axis including liver, serum and gut were found in treated rats. Hepatoxicity was observed in rats after oral exposure to TiO2 NPs at dose of 50 mg/kg bw/day with lipid peroxidation theorised to be the initial step of lipid metabolism disorder induced by TiO2 NPs due to its strong oxidative stress induction ability. No TiO2 NP accumulation was found in the liver.
Domingo, et al., 2021 – Non-sonicated study
112. To evaluate the biological response of the lung, liver, and kidneys to acute exposure to TiO2, male Wistar rats (6 animals/group) were injected with a suspension of TiO2 MP45 (average particle size: 45 μm) or TiO2 NP5 (average particle size: 5 nm) and then euthanized one month post-injection. The TiO2 used had a morphology of anatase, were lentil-shaped and formed agglomerates whereas the MPs were mostly spherical and were scattered throughout the sample.
113. Microchemical and histological analysis revealed the presence of particles, though no structural alterations, in TiO2-exposed groups. Plasma titanium concentrations were significantly higher in all TiO2-exposed groups compared to controls. An increased percentage of reactive cells (30% in the 45 μm and 20% in the 5 nm TiO2-exposed groups).
114. Significant decreases in both SOD and CAT levels were seen in lung samples of both TiO2-exposed groups, while only the TiO2-NP5 group showed decreases in both SOD and CAT levels in liver samples. No differences in either SOD or CAT activity were observed in the kidney.
115. Lipid peroxidation in lung and kidney did not differ significantly between the TiO2-exposed groups however a significant increase in oxidative damage to lipids in liver samples was observed in group TiO2-NP5.
Malakootian, et al., 2021 – Non-sonicated study
116. To investigate the effect of TiO2 NPs on DNA methylation of peripheral blood mononuclear cells (PBMCs) in vitro, blood samples from 10 human males (18 - 24 years old) were collected.
117. From these blood samples, extracted PBMCs were prepared and treated with crystalline anatase TiO2 NPs (39–74 nm) at concentrations of 0, 25, 50, 100, 150 and 200 μg/ml. The maximum non-toxic concentration of nanoparticles for PBMCs was determined by MTT assay to be 100 μg/ml (cell viability >80%). There was also a significant difference between cytotoxicity effects of TiO2 NPs between 0 and 150, and 0 and 200 μg/ml.
118. The global DNA methylation (%5-mC) in treated PBMCs revealed that TiO2 NPs could cause DNA hypomethylation in PBMCs. Exposure of PBMCs with TiO2 NPs showed that global DNA methylation (%5-mC) decreased in treated cells compared with the negative control samples. The observed %5-mC difference was −2.07 ± 1.02%. TiO2 NPs caused epigenetic alterations and DNA hypomethylation, even at the non-toxic concentrations.
Dudefoi, et al, 2021 – Non-sonicated study
119. This study aimed to investigate how TiO2 NPs, commonly used in food as a whitening agent, behave in thedigestive system and their potential impact on digestive enzymes. The researchers conducted an in vitro digestion simulation that mimicked the oral, gastric, and intestinal phases of digestion and included enzymes and appropriate fluids. The study analysed two different food-grade titanium dioxide samples (E171) and one nano-sized titanium dioxide sample (P25), The results showed that both E171 and P25 particles remained intact in the digestive fluids but formed large agglomerates, particularly in the intestinal fluid, where particles as large as 500 µm were identified.
120. The formation of these agglomerates was due to the adsorption of α-amylase and divalent cations. Pepsin was also found to adsorb onto TiO2 NPs, but only in the case of silica-covered E171. The researchers observed that in salivary conditions, TiO2 had an inhibitory effect on the enzymatic activity of α-amylase, reducing activity by up to 34% at 1 mg/mL. However, this inhibitory effect decreased to only 10% in the intestinal fluid. In the gastric phase, pepsin was not affected by any form of TiO2. Overall, the study suggests that food grade TiO2 has a limited impact on the digestion of carbohydrates and proteins, but the reduced activity observed in the oral phase requires further investigation to prevent any potential health risks associated with carbohydrate metabolism.
Li, Yan, et al, 2021 – Sonicated study
121. To study the fate of engineered TiO2 NPs in the human digestive system, a standardized food model (SFM) and simulated gastrointestinal fluids were used. Engineered TiO2 NPs were suspended in 10 mM phosphate buffer (without food matrix) and the SFM (with food matrix) at 0.5% w/w, 1% w/w and 1.5% w/w, respectively. The e-TiO2 NPs were vortexed (30 s) and then sonicated for 10 s before mixing.
122. The engineered TiO2 NPs were subjected to a three step digestion process, and their physicochemical properties were compared before and after each step. The results showed that the presence of the SFM significantly impacted the properties of the e-TiO2 NPs. The SFM acted as a dispersant and stabilized the e-TiO2 NP suspensions during each digestive phase. Therefore, it is important to use appropriate standardized food models and consider realistic physiological conditions when assessing the toxicity of ingested nanoparticles.
123. The e-TiO2 NPs underwent different transformations in their physicochemical properties after each step of the digestive process due to the pH shifts and variable concentrations of enzymes and salts in gastrointestinal fluids. These transformed e-TiO2 NPs could release titanium ions in the gastrointestinal tract. Additionally, the cell viability induced by e-TiO2 NPs was found to be strongly affected by the presence of the SFM and simulated human GI tract fluids.
Other toxicological effects
Akagi, et al., 2023 – Non-sonicated study
124. A toxicity study was carried out to assess the effects of TiO2 NPs with a crystallite size of 6 nm in rats. These sub-acute (28-day) and sub-chronic (90-day) studies were conducted in accordance with the Organisation for Economic Co-operation and Development Guidelines for the Testing of Chemicals 407 and 408, respectively. Six-week-old male and female F344/DuCrlCrlj rats were treated daily by oral gavage with 10, 100, and 1000 mg/kg bw/day (5/sex/group) for 28 days and with 100, 300, and 1000 mg/kg bw/day (10/sex/group) for 90 days.
125. Anatase-type nanosized TiO2 NPs with a crystallite diameter of 6 nm (purity 93% was suspended, with 0.2% Na2HPO4 used as a dispersant. Its administration volume was set at 10 ml/kg bw to intend to prevent aggregation.
126. In both the 28- and 90-day studies, no adverse treatment-related effects were observed in body weight, organ weight, urinalysis, haematology, or serum biochemistry.
127. In the 28-day study, TiO2 NPs were observed in the gastrointestinal lumen, nasal cavity, epithelium, and stromal tissue. They were also seen in Peyer’s patches in the ileum, cervical lymph nodes, mediastinal lymph nodes, bronchus-associated lymphoid tissue, and trachea in the 90-day study. It was noted that no inflammation, tissue injury, or other adverse biological effects were observed around the particle deposits.
128. It was found that TiO2 NPs were barely absorbed and accumulated in the liver, kidney, and spleen tissues. Immunohistochemical analysis of colonic crypts in both male and female 1000 mg/kg bw/day groups showed no extension of the proliferative cell zone or preneoplastic cytoplasmic/nuclear translocation of β-catenin.
129. Concerning genotoxicity, there was no significant increase in micro-nucleated or γ-H2AX positive hepatocytes, and no induction of γ-H2AX was seen at the particle deposit sites.
130. To conclude, after repeated oral administration of TiO2 with a crystallite size of 6 nm at up to 1000 mg/kg bw/day, no effects were observedregarding general toxicity, accumulation of titanium in the liver, kidneys, and spleen, abnormality of colonic crypts, or induction of DNA strand breaks and chromosomal aberrations.
Colin-Val, et al., 2022 - Non-sonicated study (Cardiovascular effects)
131. This study investigated food-grade titanium dioxide (E171) toxicity in rat cardiomyoblasts and hearts. E171 internalization and impact on cell viability, proliferation, mitochondria, lysosomes, F-actin distribution, and cell morphology were evaluated in H9c2 cells.
132. E171 nanoparticles of 25 µg/ml were dispersed in DMEM supplemented with 10% FBS or in saline solution. E171 suspensions were vortexed at 60 Hz for 10 minutes. The H9c2 cells were cultured in 96-well plates and exposed to 1, 5, and 25 μg/cm2 E171, proliferation and viability were evaluated after 72 hours of exposure.
133. Additionally, effects of E171 were measured on cardiac function in ex vivo rat hearts. E171 was taken up by cells and translocated into the cytoplasm. It was observed that E171 particles changed cell morphology reducing proliferation and metabolic activity.
134. Higher caspase-3 and caspase-9 expression as well as Tunel-positive cells induced by E171 exposure indicated apoptotic death. Mitochondrial and lysosome alterations resulting from mitophagy were detected after 24 and 48 h exposure, respectively. Additionally, high E171 concentrations (25 μg/cm2) caused rearrangements of the F-actin cytoskeleton. Finally, hearts exposed to E171 showed impaired cardiac function. Overall, these results support E171 toxicity in cardiac cells in vitro altering cardiac function in an ex vivo model, indicating that consumption of this food additive could be toxic and may lead to the development of cardiovascular disease.
Pogribna, et al, 2022 - Non-sonicated study (colorectal (Caco-2) and lung (NL20) epithelial cell effects)
135. The effect of TiO2 NPs exposure on histone modifications, a major epigenic mechanism, was investigated in human colorectal (Caco-2) and lung (NL20) epithelial cell lines. Histone H3 and H4 modifications were assessed by array analysis using the EpiQuickTM Histone H3 or H4 Modification Multiplex Assay after 72 hours of treatment.
136. The study found that exposure to 100 µg/ml titanium dioxide nanoparticles caused changes in the levels of 17 histone modifications in human cells. Western blot analysis confirmed changes in selected histone modifications (Caco-2 cells: H3cit, H3K9me3, H3K27me3, H3K36me3, H3K9ac, and H4K8ac; NL20 cells: H3K4me3, H3K9me3, H3K27me3, H3K9ac, and H3K18ac), and aberrant expression of histone modifying enzymes was also observed.
137. The study identified 12 genes that were affected in both cell lines, and qRT-PCR analysis confirmed the array results for several selected histone modifying enzyme genes (ASH1L, CARM1, EHMT2, HAT1, HDAC9, KMT2E, NCOA1, SETDB2, and USP16). Overall, the study demonstrated that histone modification is involved in the toxicity of TiO2 NPs and that epigenetic studies are important for assessing the potential risks of nanoparticle exposure.
Rodriguez-Ibarra, et al, 2022 – Sonicated study (Cancer effects)
138. This study investigated the effects of food-grade E171 may have on the human colon cancer cell line (HCT116) after 24 hours. The E171 had an average particle size of 100 nm and was given in the following concentrations: 0, 1, 10 and 50 μg/cm2.
139. During preparation, E171 was dispersed in 1 mL of cell culture medium and sonicated at 60 Hz for 30 min.
140. In this study, it was found that in the absence of cytotoxicity that E171 accumulated in the cancer cell line in cells are a 24 hour exposure, this lead to an increase in granularity and reactive oxygen species, inducing alterations in the molecular pattern of nucleic acids and lipids, and causing nuclei enlargement, DNA damage and tubulin depolymerization.
141. After the E171 was removed, colon cells were then cultured for a further 48 hours to analyse its ability to restore the previously detected alterations.
142. The study discovered that E171 could not reverse the changes observed in colon cells after 24 hours of being exposed. It was concluded that exposure to E171 caused alterations that cannot be reverted after 48 hours if E171 is removed from colon cells.
Alswady-Hoff, et al., 2021 – Sonicated study (Cardiovascular effects)
143. In vitro, pulmonary epithelial cells were exposed to two doses (0.96 and 1.92 µg/cm2) of TiO2 for 13 weeks and any effects on cell cycle and cell death mechanisms (apoptosis and autophagy) were determined after 4, 8 and 13 weeks of exposure. Changes in telomere length, cellular protein levels and lipid classes were also analysed at 13 weeks of exposure.
144. The TiO2-containing solution was sonicated for 16 minutes during preparation and the TiO2 particles characterised as spherical, with the majority found as aggregates or agglomerates of varying sizes.
145. TiO2 exposure was seen to increase the fraction of cells in G1-phase and reduced the fraction of cells in G2-phase, which was accompanied by an increase in the fraction of late apoptotic/necrotic cells. This corresponded with an induced expression of key apoptotic proteins i.e., BAD and BAX, and an accumulation of several lipid classes involved in cellular stress and apoptosis. Quantitative proteome profiling data also showed an increase in proteins involved in cell stress and genomic maintenance pathways following TiO2 exposure.
Ferrante, et, al., 2022 – Sonicated study (Cytotoxic effect)
146. This study investigated the DNA damage and apoptosis in colon cancer cells HCT-116 and Caco-2 induced by engineered TiO2 NPs (60 nm) and titanium dioxide food additive E171.
147. TiO2 NPs (60 nm) were weighed into 50 mL polypropylene vials, suspended in cell culture medium at a concentration of 1000 mg/L, and sonicated at 300 W for 15 min to prevent aggregation immediately before the dilutions required for cell exposure.
148. The results showed that TiO2-NPs and E171 had a cytotoxic effect on Caco-2 and HCT 116 cancer cells in a concentration-dependent manner, indicating a possibly harmful effect.
Zhao, et al., 2021 – Sonicated study (Metabolic syndrome)
149. To evaluate the stronger adverse effects of oral exposure to TiO2 NPs in a fructose-induced metabolic syndrome mouse model compared to normal mice. Metabolic syndrome is a pathological condition caused by an imbalance of carbohydrates, fats, proteins and other substances metabolised in the body.
150. During preparation, the TiO2 NPs were dispersed in 1 x PBS solution and then ultrasonicated for 30 min before use. The TiO2 NPs was reported to be anatase crystals with a spherical distribution and a diameter of ~25.2 nm.
151. Male Kunming mice (8~10 mice/group) were orally given one of the following solutions with water by gavage daily for 8 weeks: 0 mg/kg bw TiO2 NPs and 0% wt/vol fructose (Control/group 1), 20 mg/kg bw TiO2 NPs (group 2), 30% wt/vol fructose (group 3), 2 mg/kg bw TiO2 NPs and 30% fructose (group 4), or 20 mg/kg bw TiO2 NPs and 30% fructose (group 5).
152. The 2 mg/kg bw low dose of TiO2 NPs was referred to as the actual level of human exposure, with the high dose of 20 mg/kg bw being a ten-fold increase.
153. Compared to the control group, the low-dose of 2 mg/kg bw TiO2 NPs did not result in severe hepatotoxicity. Compared to the healthy non-syndrome mice, the high-dose of 20 mg/kg bw TiO2 NPs in syndrome mice was seen to induce aggravated hepatic inflammation, fibrosis, and apoptosis due to more drastic oxidative stress in the liver.
154. A significant increase in Ti and lipopolysaccharide burden were seen in the liver and serum of syndrome mice, which may have worsened portend intestinal leakage. Further tight junction-related protein expression showed that TiO2 NPs induced a further increase in serious intestinal permeability.
155. It was found that TiO2 NPs exacerbated the increase in intestinal permeability and inflammatory response through increased oxidative stress, thereby leading to more TiO2 NPs and lipopolysaccharides travelling to the liver and further aggravating liver injury in syndrome mice.
156. These findings suggest that the metabolic syndrome population, and potentially other sub-healthy populations, might require further attention in terms of the hazard of TiO2 NPs to their health.
Ogunsuyi, et al., 2022 – Sonicated study (Systemic toxicity)
157. The systemic toxicity of TiO2 NPs was investigated using male Swiss mice (5 mice/group) by dosing them intraperitoneally with TiO2 NPs (9.38, 18.75, 37.50, 75.00, and 150.00 mg/kg bw) for 5 and 10 days at 24 hour intervals. For the 10 day exposure, mice were dosed for 5 consecutive days and then were not dosed for the following 5 days in order to assess residual effects of TiO2.
158. The TiO2 NPs (<25 nm) used were anatase with a spherical shape. During preparation, TiO2 NP suspensions were sonicated in a water bath at 60 W for 10 min.
159. TiO2 NPs were found to induce systemic toxicity, including significant alterations in the haematological and biochemical parameters, with varying degrees of histopathological lesions observed in the liver, kidneys, spleen, heart, and brain of treated mice. More pronounced systemic damage was observed at 10 days than at 5 days.
160. Mice (4 mice/group) were also dosed with 150 or 300 mg/kg bw for a TiO2 NP acute toxicity study. There was no mortality or significant differences in percentage net body weight. However, after treatment with 300 mg/kg bw TiO2 NPs, mice did exhibit clinical signs including excess mucus secretion from the anus.
Fattori, et al., 2023 – Non-sonicated (Cytotoxicity)
161. The mouse fibroblast cell line (LA-9) was used to investigate the cytotoxicity of TiO2 NPs functionalized with sodium carboxylate ligands. The TiO2 NPs were anatase and suspended (0.2 mg/mL) in ultrapure water.
162. To determine nanoparticle physicochemical properties Scanning Electron Microscopy (SEM), Dynamic Light Scattering (DLS), and ATR-FTIR spectroscopy were used. After 24 hours of exposure to TiO2 NPs (50, 150, and 250 µg/mL), cell viability (MTT assay) and clonogenic survival were analysed in fibroblasts, and oxidative stress, proinflammatory state, and apoptosis were also evaluated.
163. Early and late apoptotic fibroblast cells were detected only after 24 h of exposure to 150 µg/mL TiO2 NPs. Exposure to both 150 and 250µg/mL TiO2 NPs were found to decrease fibroblast cell viability and induced ROS production. Seven days after the 24 h exposure to 250 µg/mL TiO2 NPs, a reduction of fibroblast clonogenic survival was observed. However, TiO2 NP exposure did not affect the fibroblast proinflammatory cytokines (IL-6 and TNF) secretion at any dose.
164. This study concluded that TiO2 NP photocatalytic activity likely caused an unbalanced ROS production which resulted in apoptosis, consequently reducing cell viability and metabolic activity at higher concentrations.
Questions for the Committee
165. The Committee are asked to consider the following questions:
i. What are the Committee’s views on the presented neurotoxicity studies?
ii. What are the Committee’s views on the presented immunotoxicological studies?
iii. What are the Committee’s views on reproductive toxicity?
iv. What are the Committee’s views on the absorption, distribution, metabolism and excretion (ADME) of titanium dioxide?
v. Does the committee have any other comments?
Secretariat
July 2023
List of Abbreviations and Technical Terms
|
ADI |
Acceptable Daily Intake |
|
FSA |
Food Standards Agency |
|
TiO2 |
Titanium Dioxide |
|
TiO2 NPs |
Titanium Dioxide Nanoparticles |
|
E171 |
Food-grade Titanium Dioxide |
|
GI |
Gastrointestinal |
|
EFSA |
European Food Safety Authority |
|
BW |
Body weight |
|
SOD |
Superoxide dismutase |
|
IL |
Interleukin |
|
GFAP |
Glial fibrillary acidic protein |
|
ATP |
Adenosine triphosphate |
|
TEM |
Transmission Electron Microscopy |
|
SOD |
Superoxide dismutase |
|
NSC |
Nanoscale considerations |
References:
Akagi, J.I., Mizuta, Y., Akane, H., Toyoda, T. and Ogawa, K., 2023. Oral toxicological study of titanium dioxide nanoparticles with a crystallite diameter of 6 nm in rats. Particle and Fibre Toxicology, 20(1), pp.1-23.
Alswady-Hoff, M., Erdem, J.S., Phuyal, S., Knittelfelder, O., Sharma, A., Fonseca, D.D.M., Skare, Ø., Slupphaug, G. and Zienolddiny, S., 2021. Long-term exposure to nanosized TiO2 triggers stress responses and cell death pathways in pulmonary epithelial cells. International journal of molecular sciences, 22(10), p.5349.
Al‐Doaiss, A., Jarrar, B., Shati, A., Al‐Kahtani, M. and Alfaifi, M., 2021. Cardiac and testicular alterations induced by acute exposure to titanium dioxide nanoparticles: Histopathological study. IET nanobiotechnology, 15(1), pp.58-67.
Arslan, N.P., Keles, O.N. and Gonul-Baltaci, N., 2022. Effect of titanium dioxide and silver nanoparticles on mitochondrial dynamics in mouse testis tissue. Biological Trace Element Research, 200(4), pp.1650-1658.
Bettini S, Boutet-Robinet E, Cartier C, Comera C, Gaultier E, Dupuy J, Naud N, Tache S, Grysan P, Reguer S,Thieriet N, Refregiers M, Thiaudiere D, Cravedi JP, Carriere M, Audinot JN, Pierre FH, Guzylack-Piriou L and Houdeau E (2017): Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon, Scientific Reports, 7, 40373.
Blevins, L.K., Crawford, R.B., Bach, A., Rizzo, M.D., Zhou, J., Henriquez, J.E., Khan, D.I.O., Sermet, S., Arnold, L.L., Pennington, K.L. and Souza, N.P., 2019: Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food and Chemical Toxicology, 133, p.110793.
Canli EG, Gumus C, Canli M and Ila HB (2020): The effects of titanium nanoparticles on enzymatic and non-enzymatic biomarkers in female Wistar rats, Drug and Chemical Toxicology, 1–9.
Chen, Z., Han, S., Zheng, P., Zhang, J., Zhou, S. and Jia, G., 2022. Landscape of lipidomic metabolites in gut-liver axis of Sprague–Dawley rats after oral exposure to titanium dioxide nanoparticles. Particle and Fibre Toxicology, 19(1), p.53.
Chen, Z., Han, S., Zhou, D., Zhou, S. and Jia, G., 2019. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale, 11(46), pp.22398-22412.
Chen Z, Wang Y, Zhuo L, Chen S, Zhao L, Chen T, Li Y, Zhang W, Gao X, Li P, Wang H and Jia G (2015): Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration, Nanomedicine: Nanotechnology, Biology, and Medicine, 11, 1633–1642.
Colin-Val, Z., Vera-Márquez, C.D., Herrera-Rodríguez, M.A., del Pilar Ramos-Godinez, M., López-Saavedra, A., Cano-Martínez, A., Robledo-Cadena, D.X., Rodríguez-Enríquez, S., Correa, F., Delgado‐Buenrostro, N.L. and Chirino, Y.I., 2022. Titanium Dioxide (E171) Induces Toxicity in H9c2 Rat Cardiomyoblasts and Ex Vivo Rat Hearts. Cardiovascular Toxicology, 22(8), pp.713-726.
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (2021) Meeting of the Committee on 6th July 2021. Available at : COT Final Minutes July 2021 (food.gov.uk).
Cui, Y., Che, Y. and Wang, H., 2021. Nono‐titanium dioxide exposure during the adolescent period induces neurotoxicities in rats: Ameliorative potential of bergamot essential oil. Brain and Behavior, 11(5), p.e02099.
Danafar A, Khoradmehr A, Hosseini Bondarabadi M, Mazaheri F, Tamadon A, Pourmasoumi S, Gholizadeh L, Moshrefi M, Halvaei I, Hosseini A, Golzadeh J, Rahiminia T, Anvari M. “Impairment of sperm efficiency in mice following short-term nano-titanium dioxide exposure: An experimental study,” Int J Reprod BioMed 2021; 19: 1045–1058. https://doi.org/10.18502/ijrm.v19i12.10055.
Deng, Y., Meng, X., Ling, C., Lu, T., Chang, H., Li, L., Yang, Y., Song, G. and Ding, Y., 2022. Nanosized titanium dioxide induced apoptosis and abnormal expression of blood-testis barrier junction proteins through JNK signaling pathway in TM4 cells. Biological Trace Element Research, 200(12), pp.5172-5187.
Domingo, M.G., Kurtz, M., Maglione, G., Martin, M., Brites, F., Tasat, D.R. and Olmedo, D.G., 2022. Systemic effect of TiO2 micro‐and nanoparticles after acute exposure in a murine model. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 110(7), pp.1563-1572.
Dudefoi, W., Rabesona, H., Rivard, C., Mercier-Bonin, M., Humbert, B., Terrisse, H. and Ropers, M.H., 2021. In vitro digestion of food grade TiO2 (E171) and TiO 2 nanoparticles: Physicochemical characterization and impact on the activity of digestive enzymes. Food & Function, 12(13), pp.5975-5988.
Ebrahimzadeh BA, Mohammadipour A, Fazel A, Haghir H, Rafatpanah H, Hosseini M and Rajabzadeh A (2017): Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis, Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie, 69, 329–337.
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food) (2016): Re-evaluation of titanium dioxide (E 171) as a food additive, EFSA Journal 2016;14(9):4545, 83, doi: Re‐evaluation of titanium dioxide (E 171) as a food additive - - 2016 - EFSA Journal - Wiley Online Library.
EFSA FEEDAP (2021a): Safety and efficacy of a feed additive consisting of titanium dioxide for all animal species (Titanium Dioxide Manufacturers Association), EFSA Journal 2021;19(6):6630, doi: https://doi.org/10.2903/j.efsa.2021.6630.
Fattori, A.C.M., Brassolatti, P., Feitosa, K.A., Pedrino, M., Correia, R.D.O., Albuquerque, Y.R., Rodolpho, J.M.D.A., Luna, G.L.F., Bernardi, J.C., Zucolotto, V. and Speglich, C., 2021. Titanium dioxide nanoparticle (TiO2 NP) induces toxic effects on LA-9 mouse fibroblast cell line. Cellular Physiology and Biochemistry, 57(2), pp.63-81.
Ferrante, M.; Grasso, A.; Salemi, R.; Libra, M.; Tomasello, B.; Fiore, M.; Copat, C. DNA Damage and Apoptosis as In-Vitro Effect Biomarkers of Titanium Dioxide Nanoparticles (TiO2-NPs) and the Food Additive E171 Toxicity in Colon Cancer Cells: HCT-116 and Caco-2. Int. J. Environ. Res. Public Health 2023, 20, 2002. https://doi.org/10.3390/ijerph20032002.
Gerber, L.S., Heusinkveld, H.J., Langendoen, C., Stahlmecke, B., Schins, R.P. and Westerink, R.H., 2022. Acute, sub-chronic and chronic exposures to TiO2 and Ag nanoparticles differentially affects neuronal function in vitro. Neurotoxicology, 93, pp.311-323.
Grissa, I., Guezguez, S., Ezzi, L., Chakroun, S., Sallem, A., Kerkeni, E., Elghoul, J., El Mir, L., Mehdi, M., Cheikh, H.B. and Haouas, Z., (2016): The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environmental Science and Pollution Research, 23, pp.20205-20213.
Grissa, I., ElGhoul, J., Mrimi, R., El Mir, L., Cheikh, H.B. and Horcajada, P., (2020): In deep evaluation of the neurotoxicity of orally administered TiO2 nanoparticles. Brain research bulletin, 155, pp.119-128.
Han HY, Yang MJ, Yoon C, Lee GH, Kim DW, Kim TW, Kwak M, Heo MB, Lee TG, Kim S, Oh JH, Lim HJ, Oh I, YoonS and Park EJ (2020): Toxicity of orally administered food-grade titanium dioxide nanoparticles, Journal of Applied Toxicology, https://doi.org/10.1002/jat.4099.
Hashem MM, Abo-El-Sooud K, Abd-Elhakim YM, Badr YA, El-Metwally AE,Bahy-El-Dien A (2020): The long-termoral exposure to titanium dioxide impaired immune functions and triggered cytotoxic and genotoxic impacts in rats, Journal of Trace Elements in Medicine and Biology, 60.
Kamal, Z., Ebnalwaled, A.A., Al-Amgad, Z., Saied, A.A., Metwally, A.A. and Said, A.H., 2023. Immunomodulatory and antioxidant effect of green synthesized titanium dioxide nanoparticles on pregnant female albino rats and their fetuses. Environmental Science and Pollution Research, 30(19), pp.55455-55470.
Kandeil MA, Mohammed ET, Hashem KS, Aleya L and Abdel-Daim MM (2019): Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability, Environmental Science and Pollution Research International.
Korábková, E.; Kašpárková, V.; Jasenská, D.; Moricová, D.; Dad’ová, E.; Truong, T.H.; Capáková, Z.; Vícha, J.; Pelková, J.; Humpolíˇcek, P. Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. Int. J. Mol. Sci. 2021, 22, 10614. https://doi.org/10.3390/ijms221910614.
Li, J., Yang, S., Lei, R., Gu, W., Qin, Y., Ma, S., Chen, K., Chang, Y., Bai, X., Xia, S. and Wu, C., 2018. Oral administration of rutile and anatase TiO 2 nanoparticles shifts mouse gut microbiota structure. Nanoscale, 10(16), pp.7736-7745.
Li, S., Yan, D., Huang, C., Yang, F. and Cao, Y., 2022. TiO2 nanosheets promote the transformation of vascular smooth muscle cells into foam cells in vitro and in vivo through the up-regulation of nuclear factor kappa B subunit 2. Journal of Hazardous Materials, 424, p.127704.
Li XB, Zhang YS, Li B, Cui J, Gao N, Sun H, Meng QT, Wu SS, Bo JZ, Yan LC, Wu J, Chen R (2019): Prebiotic protects against anatase titanium dioxide nanoparticles-induced microbiota-mediated colonic barrier defects, Nanoimpact, 14, 9.
Malakootian, M., Nasiri, A., Osornio-Vargas, A.R. and Faraji, M., 2021. Effect of titanium dioxide nanoparticles on DNA methylation of human peripheral blood mononuclear cells. Toxicology Research, 10(5), pp.1045-1051.
Mancuso, F., Arato, I., Di Michele, A., Antognelli, C., Angelini, L., Bellucci, C., Lilli, C., Boncompagni, S., Fusella, A., Bartolini, D. and Russo, C., 2022. Effects of titanium dioxide nanoparticles on porcine prepubertal sertoli cells: An “in vitro” study. Frontiers in Endocrinology, 12, p.1756.
Murugadoss, S.; Godderis, L.; Ghosh, M.; Hoet, P.H. Assessing the Toxicological Relevance of Nanomaterial Agglomerates and Aggregates Using Realistic Exposure
In Vitro. Nanomaterials 2021, 11, 1793. https://doi.org/10.3390/nano11071793.
Mohamed HR (2015): Estimation of TiO2nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice, Food and Chemical Toxicology: AN International Journal Published for the British Industrial Biological Research Association, 83, 76–83
Naima, R., Imen, M., Mustapha, J., Hafedh, A., Kamel, K., Mohsen, S. and Salem, A., 2021. Acute titanium dioxide nanoparticles exposure impaired spatial cognitive performance through neurotoxic and oxidative mechanisms in Wistar rats. Biomarkers, 26(8), pp.760-769.
Ni, D.Q., Ma, D.D., Hao, S.L., Yang, W.X., Kovacs, T. and Tan, F.Q., 2021. Titanium dioxide nanoparticles perturb the blood-testis barrier via disruption of actin-based cell adhesive function. Aging (Albany NY), 13(23), p.25440.
Ogunsuyi, O., Ogunsuyi, O., Akanni, O., Alabi, O., Alimba, C., Adaramoye, O., Cambier, S., Eswara, S., Gutleb, A.C. and Bakare, A., 2022. Physiological and histopathological alterations in male Swiss mice after exposure to titanium dioxide (anatase) and zinc oxide nanoparticles and their binary mixture. Drug and Chemical Toxicology, 45(3), pp.1188-1213.
Rahnama S, Hassanpour A, Yadegari M, Anvari M and Hosseini-sharifabad M (2020): Effect of titanium dioxide nanoparticles on the stereological parameters of the dentate gyrus and the morphology of granular hippocampal neurons in mice, International Journal of Morphology, 38, 1623–1630.
Riedle S, Wills JW, Miniter M, Otter DE, Singh H, Brown AP, Micklethwaite S, Rees P, Jugdaohsingh R, Roy NC,Hewitt RE and Powell JJ (2020): A murine oral- exposure model for nano- and micro-particulates: demonstrating human relevance with food-grade titanium dioxide, Nano-Micro Small, 16, 2000486.
Rodríguez-Ibarra, C., Medina-Reyes, E.I., Déciga-Alcaraz, A., Delgado-Buenrostro, N.L., Quezada-Maldonado, E.M., Ispanixtlahuatl-Meráz, O., Ganem-Rondero, A., Flores-Flores, J.O., Vázquez-Zapién, G.J., Mata-Miranda, M.M. and López-Marure, R., 2022. Food grade titanium dioxide accumulation leads to cellular alterations in colon cells after removal of a 24-hour exposure. Toxicology, 478, p.153280.
Pinget, G., Tan, J., Janac, B., Kaakoush, N.O., Angelatos, A.S., O'Sullivan, J., Koay, Y.C., Sierro, F., Davis, J., Divakarla, S.K. and Khanal, D., 2019. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Frontiers in Nutrition, 6, p.57.
Pogribna, M., Word, B., Lyn-Cook, B. and Hammons, G., 2022. Effect of titanium dioxide nanoparticles on histone modifications and histone modifying enzymes expression in human cell lines. Nanotoxicology, 16(4), pp.409-424.
Said, A.A., Nasr, Y., Galal, A.A., Abdelhamid, A.E., Mohamed, H.A., Metwally, M.M., Said, M.A., Nassan, M.A., Dahran, N. and Mohamed, A.A.R., 2022. Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life, 12(5), p.639.
Sitia, G., Fiordaliso, F., Violatto, M.B., Alarcon, J.F., Talamini, L., Corbelli, A., Ferreira, L.M., Tran, N.L., Chakraborty, I., Salmona, M. and Parak, W.J., 2022. Food-Grade Titanium Dioxide Induces Toxicity in the Nematode Caenorhabditis elegans and Acute Hepatic and Pulmonary Responses in Mice. Nanomaterials, 12(10), p.1669.
Sofranko, A., Wahle, T., Heusinkveld, H.J., Stahlmecke, B., Dronov, M., Pijnenburg, D., Hilhorst, R., Lamann, K., Albrecht, C. and Schins, R.P., 2021. Evaluation of the neurotoxic effects of engineered nanomaterials in C57BL/6J mice in 28-day oral exposure studies. NeuroToxicology, 84, pp.155-171.
Talamini L, Gimondi S, Violatto MB, Fiordaliso F, Pedica F, Tran NL, Sitia G, Aureli F, Raggi A, Nelissen I, Cubadda F,Bigini P and Diomede L (2019): Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology, 13, 1087–1101.
Urrutia-Ortega IM, Garduno-Balderas LG, Delgado-Buenrostro NL, Freyre-Fonseca V, Flores-Flores JO, Gonzalez-Robles A, Pedraza-Chaverri J, Hernandez-Pando R, Rodriguez-Sosa M, Leon-Cabrera S, Terrazas LI, vanLoveren H, Chirino YI (2016) :Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model, Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 93, 20–31.
Violatto, M.B.; Sitia, G.; Talamini, L.; Morelli, A.; Tran, N.L.; Zhang, Q.; Masood, A.; Pelaz, B.; Chakraborty, I.; Cui, D.; et al. Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents. Nanomaterials 2023, 13, 1174. Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents - PubMed (nih.gov).
Yao, L., Chen, L., Chen, B., Tang, Y., Zhao, Y., Liu, S. and Xu, H., 2021. Toxic effects of TiO2 NPs in the blood-milk barrier of the maternal dams and growth of offspring. Ecotoxicology and Environmental Safety, 208, p.111762.
Yu X, Hong F and Zhang YQ (2016): Cardiac inflammation involving in PKCe or ERK1/2-activated NF-jB signalling pathway in mice following exposure to titanium dioxide nanoparticles, Journal of Hazardous Materials, 313, 68–77.
Zhao, Y., Tang, Y., Liu, S., Jia, T., Zhou, D. and Xu, H., 2021. Foodborne TiO2 nanoparticles induced more severe hepatotoxicity in fructose-induced metabolic syndrome mice via exacerbating oxidative stress-mediated intestinal barrier damage. Foods, 10(5), p.986.
Zhou Y, Hong F, Tian Y, Zhao X, Hong J, Ze Y and Wang L (2017): Nanoparticulate titanium dioxide-inhibited dendritic development is involved in apoptosis and autophagy of hippocampal neurons in offspring mice, Toxicology Research, 6, 889–901.
Zhang S, Jiang X, Cheng S, Fan J, Qin X, Wang T, Zhang Y, Zhang J, Qiu Y, Qiu J, Zou Z and Chen C, 2020. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Archives of Toxicology, 94, 1173–1190.
Secretariat
July 2023
TOX/2023/32 - Annex A
Search strategy
The database Lit-fetch was used to search the following search terms between the dates 2021-01-01 to 2023-04-28:
|
Annex |
Search terms |
Number of hits |
Relevant hits |
|
Search "In Vitro Techniques"[Mesh] OR "Caco-2 Cells"[Mesh] OR "Lysosomes"[Mesh] OR "Hypoxanthine Phosphoribosyltransferase"[Mesh] OR "Autopsy"[Mesh] OR "Micronucleus Tests"[Mesh] OR "Pathology"[Mesh] OR Autops*[tiab] OR histopath*[tiab] OR "in vivo"[tiab] OR "in vitro"[tiab] OR GALT[tiab] OR Caco 2 cell*[tiab] OR Colonic Epithelial Cell*[tiab] OR Colorectal epithelial cell*[tiab] OR Comet assay*[tiab] OR HPRT[tiab] OR "Hypoxanthine Phosphoribosyltransferase"[tiab] OR "hypoxanthin phosphoribosyl transferase"[tiab] OR Lysosom*[tiab] OR "M cell"[tiab] OR "M cells"[tiab] OR "Microfold cell"[tiab] OR "Microfold cells"[tiab] OR Micronucleus assay*[tiab] OR Micronucleus test*[tiab] OR "read across"[tiab] OR SCGE assay*[tiab] OR single-cell gel electrophoresis assay*[tiab] OR "xanthine guanine phosphoribosyl transferase"[tiab] OR "xanthine guanine phosphoribosyltransferase"[tiab] OR xprt[tiab] AND titanium dioxide |
322 |
19 |
|
|
Search "Aneugens"[Mesh] OR "Aneuploidy"[Mesh] OR"apoptosis"[Mesh] OR "Chromatids"[Mesh] OR "Chromosomes"[Mesh] OR "DNA Damage"[Mesh] OR "DNA Repair"[Mesh] OR "Mutagens" [Pharmacological Action] OR "Mutagens"[Mesh] OR "Mutagenesis"[Mesh] OR "Oxidative Stress"[Mesh] OR Aneugen*[tiab] OR Aneuploid*[tiab] OR Chromatid*[tiab] OR Chromosome*[tiab] OR Clastogen*[tiab] OR "DNA binding"[tiab] OR DNA fragmentation*[tiab] OR DNA damage*[tiab] OR "DNA lesion"[tiab] OR "DNA lesions"[tiab] OR "DNA repair" [tiab] OR genotox*[tiab] OR mutagen*[tiab] OR Mutat*[tiab] OR oxidative stress*[tiab] AND titanium dioxide |
209 |
16 |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND toxicology) |
1 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND genotoxicity) |
8 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND human health) |
13 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND adverse effects) |
7 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND reproductive toxicity) |
5 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND developmental toxicity) |
2 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND Distribution) |
8 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND Metabolism) |
4 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND Excretion) |
1 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND ADME) |
1 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND immunotoxicology) |
0 |
No Details |
|
|
(Titanium dioxide AND Nanoparticles AND E171 AND neurotoxicology) |
0 |
No Details |
Paper titles and abstracts were manually sorted, and papers deemed not relevant to this current paper were disregarded. Full list of search results can be found in links in the above table.